1.
Title of the Technology: Peptide
based therapeutics against Parkinson’s disease
2. Value
Proposition: The peptide treatment has shown
beneficial effects in mice model of Parkinson’s disease. It decreased the
α-synuclein aggregate burden in mice brain, and improved locomotory activity.
Thus the peptide has potential to be First-in-Class therapeutics against
Parkinson’s disease.
3.
Current Technology Readiness Level (TRL) : TRL-4
4.
Brief description (max 150 words): Parkinson’s
disease (PD) is the second most prevalent
neurodegenerative disease after Alzheimer’s disease. Though multifactorial, the
accumulation of α-synuclein amyloid aggregates, known as Lewy bodies, in
dopaminergic cells of substantia nigra region of the brain is a characteristic
cellular hallmark of PD. The condition predominantly affects muscle movements manifesting
first with difficulties in chewing and swallowing, drooling, and constipation.
The disease progresses with common motor symptoms such as difficulties in
movement, tremor, rigidity and posture, etc. As of now only symptomatic relief
is available, and there is no cure.
We recently
screened various peptide based molecules and identified a potent peptide that
reduced α-synuclein amyloid aggregation in both in vitro and in vivo models of
PD.
5.
Salient Features/Advantages:
ü First-in-class
peptide based inhibitor of α-synuclein
aggregation is discovered.
ü The
molecule inhibits α-Synuclein
aggregation in sub-stoichiometry concentrations.
ü Another
cyclic-derivative of the identified also inhibited α-synuclein amyloid aggregation.
ü The
identified peptide improved locomotory activity in C. elegans model of
PD
ü The
identified peptide improved locomotory activity in mice models of PD.
ü The
peptide is known to cross blood brain barrier.
ü The
peptide reduces the pathogenic aggregate burden in mice brain.
ü The
peptide reduces Lewy neurites burden, reduces neuroinflammation, improves
tyrosine hydroxylase expression in mice brain.
6. Any
patents/copyrights & status: Patent
has been filed as PCT (PCT/IN2023/050274)
7.
Supporting publications, if any: In
progress
8.
Photographs of prototype/publication if any:
TRL: 5
Beta-lactam antibiotics are the cornerstones of antimicrobial chemotherapy. However, antimicrobial resistance against these life-saving drugs is a major public health problem worldwide. Resistance to beta-lactam antibiotics among Gram-negative bacteria is mainly due to the production of beta-lactamases, which inactivate these life-saving drugs. Bacteria achieves resistance by these challenging chemical reactions with two types of enzymes: serine β-lactamases and/or metallo-β-lactamases (MBL). Recently few serine beta-lactamase inhibitors has been approved, but to date, not a single MBL inhibitor is approved or in the clinic. So, there is an urgent unmet medical need to discover a new ideal MBL inhibitor, which can be use as adjuvant with FDA-approved beta-lactam antibiotics and would be beneficial in difficult to treat hospital-based infections.
The salient features of this technology are:
·
First-in-class novel small molecule MBL inhibitor is
discovered.
·
Discovered novel compounds showed IC50 in nano-molar
range activity against more clinical relavance pathogens NDM-1, VIM-1 and
IMP-1.
·
Compounds showed synergy with last-resort antibiotics (Meropenem and
Imipenem) in carbapenem-resistant strain.
·
Library of compounds prepared, tested against NDM-1, VIM
and IMP-1 gene, evaluated synergy, and analysed structure-activity relationship
(SAR).
·
Novel small molecule inhibitor can be use as
adjuvant with antibiotic to address difficult to treat hospital-acquired /
Carbapenem-Resistant Enterobacteriaceae (CRE) infections.
·
The lead compounds showed good physio-chemical properties
(logD and solubility)
·
In vitro ADME and toxicity data showed promising
results.
·
In preliminary in
vivo animal study, lead compound showed efficacy in thigh infection model
in combination with last resort antibiotic (Imipenem).
·
Low cost
synthesis and process optimized for the lead compounds.
·
Detailed PK/PD
studies in progress
Value Proposition:
AMR is a global unmet health problem. The
solutions developed i.e. effective novel pan-MBLs inhibitors have the potential
to be sold globally hence have high potential commercial value. Considering
fewer ongoing efforts and huge unmet medical need, many big and small
pharmaceutical industries (national and international), will be interested to
in-license any potential molecule showing promise in treating AMR infections.
Patents/copyrights:
·
WO2022/254464: SUBSTITUTED
TRICYCLIC HETEROCYCLIC COMPOUNDS AS METALLO-BETA-LACTAMASE INHIBITOR; PCT is
live, will be filing in different countries.
·
Another two series patent application filling is in progress.
The technology offers rapid and sensitive colorimetric detection of pathogens in water based on metal nanoparticle-enzyme interaction. The intensity of color development depends upon the amount of bacterial contamination present in water sample. This novel field-friendly innovation provides instant visible color change seen via naked-eye with a sensitivity of 103 cells.mL−1. The technique involves a polymer or bioreceptor coated silver nanoparticles combining catalytic activity of an enzyme that produces a colored end-point readout signal. The salient feature of the bioassay includes the co-presence of enzyme inhibitor and bioreceptor simultaneously on the surface of nanoparticles. If water is contaminated, the nanobioprobe gets entangled with microbe and enzyme is rendered free in solution consequently its catalytic activity produces pink color. On the other hand if water is microbe-free, the nanobioprobe inhibits the activity of enzyme producing yellow color. The dual activity of nanobioprobe has been demonstrated by three versions of the bioassay that are reproducible and are IPR protected. The most important advantage offered by the technology is its cost-effective, user friendly, rapid and onsite and has current Technology Readiness Level (TRL) 4.
Patents/copyrights & status: A pH based colorimetric bioassay method for bacterial detection in water by naked. Inventors: Vijayender Bhalla, Pargat Singh, Saloni kakkar, Bharti, Virendra Nishad. Application No. 201811035461; 20/09/2018. Published patent; 14/08/2020.
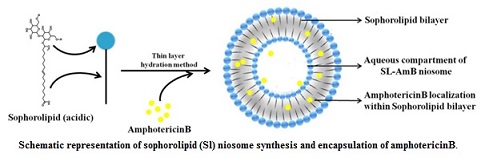
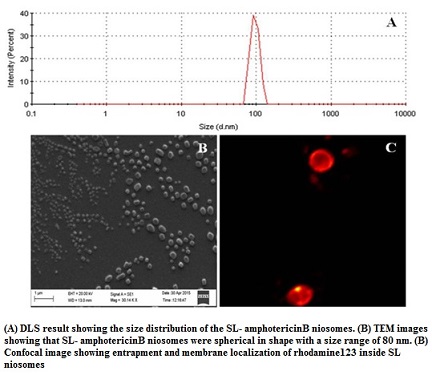
Efficient and safer delivery of drugs (with low solubility and poor bioavailability) and is a challenging task in pharmaceutical and biotechnological industries. Niosomes, by virtue of its vesicular structure, can house both hydrophilic and hydrophobic molecules within its compartments. By exploring the structure-forming attributes of acidic sophorolipid (a microbial glycolipid) we have developed a carrier system for drug and biomolecule delivery. The versatile system can be synthesized with different charges (+Ve/-Ve), size and physical characteristics. They are highly suitable for the delivery of drugs having poor bioavailability due to low water solubility. A prototype of the system has been tested for the delivery of poorly soluble drug amphotericin B. This preparation can also be used for gene and protein delivery and for bio-imaging purposes.
 |
 |
Sophorolipid is an important microbial glycolipid biosurfactant, containing a sugar moiety (sophorose) and a fatty acid and is available in lactonic and open chain acidic form. These molecules have versatile application in cosmetic and pharmaceutical industries along with its application as detergent and cleaning agent. Sophorolipid is known to be a less-toxic, non-alergic biosurfactant with significant biomedical potential as anticancer, virucidal, bactericidal and antifungal agents. These molecules have been approved for external application by the USFDA and are used in the shampoo, body washes, perfume and fragrance products in the cosmetic and body care industries. We have developed a cost effective technology for the production of sophorolipid using agri-industry by product. The technology is ready at bench scale and has scope of development at the pilot scale.
Working with the shape-function properties of gelsolin, our and others work led to conclusion that plasma gelsolin levels aid in body’s ability to recover from injuries, both physical and biochemical. A funding pitch made to Gates Foundation to correlate plasma gelsolin levels in mothers’ with their uninduced or spontaneous gestation time was granted in 2013. Research led to formulation of an estimation for plasma gelsolin based on patented aptamers and protocol to coat them on ELISA plates. The prototype was first tested in samples from PGI Chandigarh whichindicated plasma gelsolin is lower in mothers delivering prematurely. Further research done with plasma samples from Rajasthan, Gujrat and Madhya Pradesh provided by Oniosome Healthcare Pvt. Ltd. also showed that our method and mathematical model can predict uninduced birth prediction time with about 85% accuracy in the fifth month of singleton gestation.
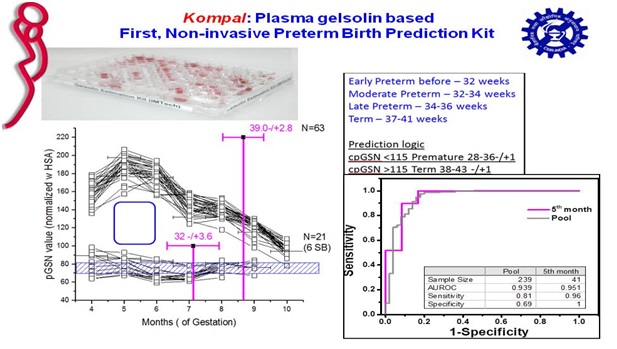
Patents/copyrights: Aptamers for purifying and quantifying gelsolin and its variants . WO2016056028. ALIVE in PCT; Positive Examination; Granted in many zones
A process for the preparation of bio-organic coated gold and silver nanoparticles using blue light. WO2016157220. ALIVE in PCT; Positive Examination; Granted in some zones
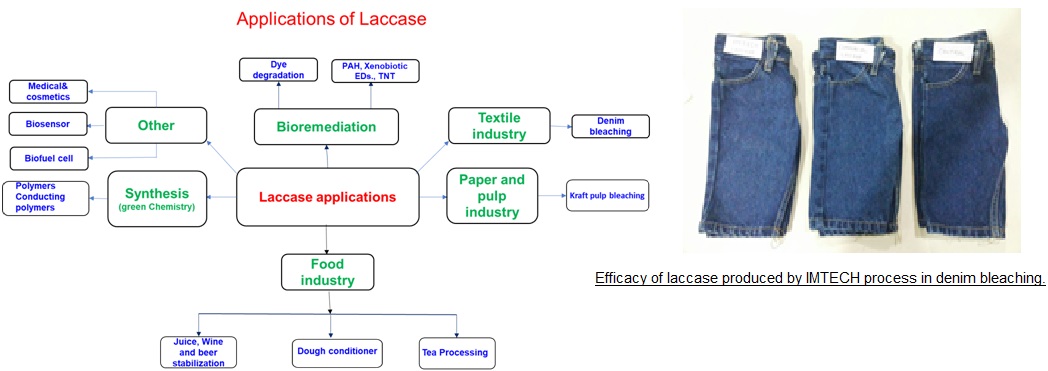
A process has been developed for production of a novel high redox potential laccase using a fungal strain isolated in CSIR-IMTECH. Laccase fermentation is carried out in a special surface culture tray reactor. The laccase produced is extracellular and easy to purify from the broth. Laccase fermentations generally have extended duration making it expensive to run. The CSIR-IMTECH laccase fermentation process is low energy intensive and based on moderate cost not complex medium. The process has been demonstrated on 10 L scale and has yield of 15,000 IU per liter.(1 unit of enzyme activity is the enzyme quantity that produce 1 μM of ABTS cation per min in 50 mM Citrate phosphate buffer, pH 4.0 at 300C and 5 minute incubation time. ). The process can be easily scaled up to semi pilot or pilot scale.
Advantages/Value Proposition: Laccase (EC 1.10.3.1) has broad enzyme specificity that imparts high application potential for its applications in various fields such as bioremediation, textile, pulp and paper , food and beverage, cosmetics and organic synthesis. Laccase production is difficult as it is produced in the idiophase when cell metabolism is slow. Low intrinsic production and long fermentation cycle increase laccase production cost.
The CSIR-IMTECH process has addressed some issues in the production of laccase and using a novel strain, a reliable process for the production of high redox potential laccase has been developed. The laccase has been tested in industrial environment and has been found to give good performance in denim bleaching.
Applications:
 Patents/copyrights:Method for obtaining laccase enzyme from Arthrographis sp. PCT Patent Appn,No.WO 2012023021 A1
Patents/copyrights:Method for obtaining laccase enzyme from Arthrographis sp. PCT Patent Appn,No.WO 2012023021 A1
Chemotherapy constitutes an indispensable component of the cancer treatment. Since chemotherapeutic drugs are cytotoxic and one of the critical side effects of chemotherapy is myelosuppression associated neutropenia. Chemotherapy induced neutropenia could predispose cancer patients to fatal infections and multi-organ failure. Clinical management of neutropenia with administration of Granulocyte-Colony Stimulating Factor (G-CSF) has played an instrumental role in improving the outcome of chemotherapy. However, administration of G-CSF leads to escalation in the cost of cancer management. G-CSF is also used for patients suffering from leucopenia, AIDS, sepsis and in bone-marrow transplantation.Furthermore, current research indicates multiple roles of G-CSF as a safe and efficacious drug, with potential of treating diseases beyond neutropenia. According to recent market survey, it is estimated that because of increasing demand of G-CSF, the market value of G-CSF would double in next five years. In the light of this information, we have developed G-CSF biosimilar and PEGylated G-CSF biosimilar. The G-CSF biosimilar technology is ready at the shake-flask level and we are working to develop the process for high-density fermentation. PEGylated G-CSF has longer half-life and is administered once per cycle of chemotherapy.
Technology Package: The engineered construct for E. coli expression, process for purification, characterization and in vitro bioactivity assay, in vivo bioactivity assay and hands-on training to people, to perform the same. PEG conjugation reaction conditions and purification of PEGylated G-CSF, characterization and in vitro and in vivo bioactivity assay and hands-on training.
Patents/copyrights: A polypeptide exhibiting granulocyte-colony stimulating factor activity. Application number PCT/IN2018/050873.
The major share of the biotherapeutic market belongs to the monoclonal antibodies (mAbs) and recombinant proteins. Among all therapeutic mAbs, adalimumab which is most widely used as safe and effective therapeutic option for rheumatoid arthritis (RA) patients is world’s best selling drug. However, adalimumab-based therapy is very expensiveand therefore out of reach for a large population. Therefore, generating biosimilar of adalimumab (known to be considerably cheaper) is the need of the hour to meet the larger societal demand. In recent years, we have developed a promising CHO cell clone (IMT_C11), which produces anti-TNFα mAbwell within industry acceptable range. Our preliminary results demonstrate biosimilarity of IMT_C11 mAb with the originator molecule, adalimumab. For example, the originator molecule adalimumab and anti-TNFαmAb produced by IMT_C11 clone shows comparable TNFα neutralizing ability. Furthermore, preliminary biophysical characterization along with the mass spectrometric analysis confirms IMT_C11 mAb to be similar to adalimumab.
Typhoid is a life threatening infection caused by the salmonella entericaserovarTyphi and currently emerging as a major public health problem in developing countries. Diagnosis of Salmonella Typhi is quite challenging because the symptoms of typhoid fever overlap with a wide range of other diseases. The conventional methods which include the blood culture and antibody based detection are considered as a gold standard for the diagnostic of typhoid fever. These methods often give rise to false negative results because of the prior intake of antibiotics and hence underestimates the actual occurrence of disease. Therefore the need of an hour is to develop a fast, rapid and sensitive immunoassay to detect the Salmonella. By considering all these point our technology focuses on the invention of hand-held opticall system by employing three highly selective biomarkers CdtB, Vi and PagC specific to S. Typhi for the detection of typhoidalsalmenollosis. The test would be more specific and accurate by providing serological detection of antibodies (IgG/IgM ) against these three biomarkers in typhoid infected patients. Thus the current technology can be useto provide a rapid, sensitive and reliable immunoassay platform for detection of typhoid fever.
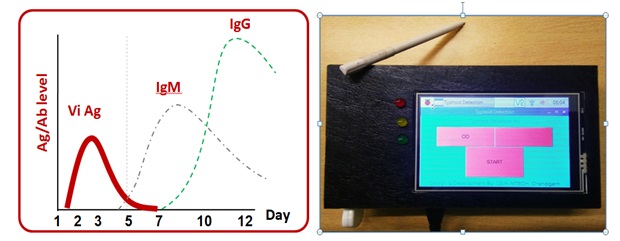
Patents/copyrights: IP is filed
Due to the advanced technologies in printing, scanning and communication devices, counterfeiters still able to make fake currency notes and counterfeit the original documents. Branded products were also duplicated by the counterfeiters using advanced colour copier machines. Existing colour shift inks, optically variable inks contains toxic organic, inorganic chemicals and pigments uses optical reflective effects to show the colour change when tilted at certain angle or exposed to UV detectors. In our lab we developed the anticopier and difficult to imitate colour shift Laminated protein film. The environmental friendly Biodegradable, non toxic, Microbial protein ink was deposited on Lamination temperature resistance porous membrane forms a stable thin protein film which will not tear easily. The laminated protein film shows reversible colour change from purple to yellow under illumination and yellow to purple under non-illumination condition with in few seconds. This reversible colour change under Illumination can be triggered by using Handy torch light , Handy green laser pointers, Handy Mobile phone flash light and also under normal sunlight. The reversible colour change from purple to yellow and yellow to purple was identified by naked eye without using external reader instruments. This Laminated protein film with reversible colour changing property can be used in developing high security feature Holograms.

We entered CDA agreement with Jupiter Technologies Pvt Ltd, Bangalore for further development of protein film based High security applications.
Pullulan, a homo-polysaccharide of maltotriose, is an extremely versatile ingredient with capability of providing a technology platform for product innovation.Due to unique physicochemical properties, pullulan has found applications in food, pharmaceuticals and cosmetic industries. Pullulan can be formed into capsules for use with pharmaceutical and nutraceutical products. Its non-animal origin and GRAS status ensures safety and acceptability across diverse consumer groups. The global market size of pullulan was 10,000 TPA in 2009 (CAGR : 6.75%).
In CSIR-IMTECH, we have developed a process for pullulan production using osmotolerant strain of Aureobasidium pullulans. The process isready for demonstration at 500L fermenter scale. The yield and productivity of our process is higher as compared to published reports. Moreover, the process was developed using low-cost agri-industrial residues and would result in significant reduction in the RMCvis a vis cost of production. Hence, our process is actually about creating wealth from waste.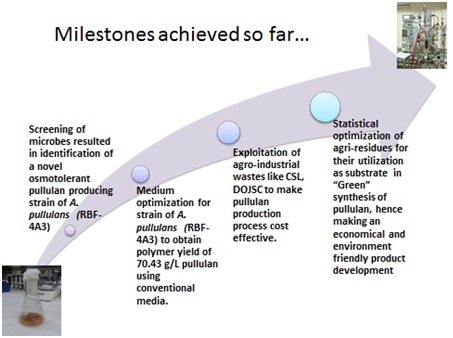
You tube link: https://www.youtube.com/watch?v=NTAnuen-bnQ
Bacterial skin infections is one of the serious problems and if it is not treated at appropriate time, spreads secondary infections which is sometimes more fatal. To avoid such situations, effective and fast acting formulation is needed which is possible by combination therapy with currently used FDA approved antibiotics. Most of the antibiotics do not absorb rapidly due to their hydrophobic nature and these are obsolete. To make the use of all such antibiotics in better fashion, such options are important.There is an unmet medical need for novel topical formulations for safe and efficacious delivery of biological therapeutics across the skin leading to achieve better long-term outcomes with fewer side effects. The formulation which can deliver antibacterial across the skin more effectively than available therapy is a goal of this technology. Development of formulation, quality control parameters and determination of effective dose in approved animal model will be the component of this technology. The salient features of this technology are:
Patents/copyrights: Proof of concept and findings are patented. The patent application has been filed for demonstration of proof of concept for delivery of antibiotic/drugs, etc. Also the work has been published. Currently, we are working on increasing the spectrum of activity of IMT-P8 with FDA approved antibiotics, preferably used in topical, skin and wound infection by Gram positive bacteria and further appropriate formulation development based on the in vitro and in vivo results. The research in this regard is underway.
The rise in extensively drug-resistant (XDR) and pan-drug-resistant (PDR) K. pneumoniae worldwide is a concerning threat as the infections are untreatable in some cases. Polymyxins considered as rescue antibiotics to treat deadly infections caused by Gram-negative bacteria, are becoming ineffective due to emergence of resistant strains. M152-P3 isolated in our lab is highly active against colistin-resistant clinical isolates of K. pneumoniae. A drug specifically active against K. pneumoniae strains of clinical relevance may be used as a definitive therapy. The analogues of this compound which are more efficacious and less toxic can be patented and commercialized. Moreover, the technology for producing this natural compound using the producer microorganism itself in fermentation after media and process optimization can have commercial value. Alternatively, the biosynthetic genes forM152-P3 compounds can be cloned and expressed in a suitable host for large scale synthesis at cheaper scale. The salient features of this technology are:
The efflux pump (EP) is one of the major factors to develop antibiotic resistance in bacteria. There is not a single efflux pump inhibitor (EPI) which is in clinical practice.So, it is need of hour to discover new ideal EPI which can be used in combinations with FDA approved antibiotics. The salient features of this technology are:
Current Status: Lead optimization and Pre-clinical development is undergoing
Taking cue from literature that there is a correlation between inner structural organization of keratin and lipid in hair, and presence/absence of breast cancer, we explored if small angle X-ray scattering (SAXS) can distinguish between hair from confirmed cancer patients and relatives lacking any cancerous malady. Results brought forth that significantly altered SAXS profile is seen in cases of cancer, tuberculosis, PCOD and NAFL compared to normal ones. We developed programs for automated data collection, analysis and interpretation. Our methodology has been confirmed with about 280 clinically validated cases in India. Recently, we are working on reducing the cost of the analysis instrument, so that our diagnosis can be provided at a lower cost. Research was supported by Grand Challenge Program and CSIR, and now we are open to collaborations for third party validation leading towards regulatory clearances.
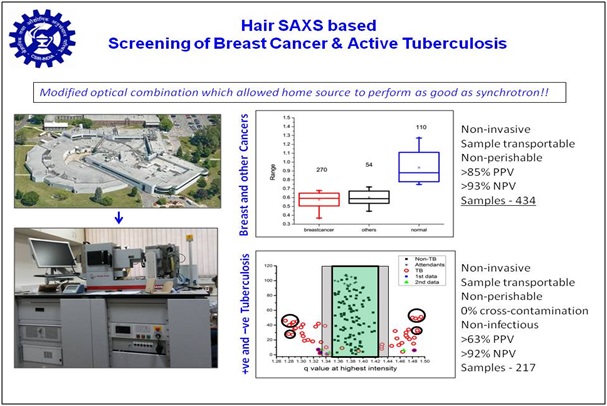
1. patents/copyrights & status:
In vitro method for detecting active mycobacterium tuberculosis using hair small angle x-ray scattering profile
WO201896557
ALIVE in PCT; Report 2017-11-21 Positive Examination; Examination WO/A1 on 2018-05-31 and WO/DPE2 2018-07-05
Two Provisional Patents filed on screening for Cancer
PCT full filing due in March 2019, and Oct 2020
1. Taking cue from literature that there is a correlation between inner structural organization of keratin and lipid in hair, and presence/absence of breast cancer, we explored if small angle X-ray scattering (SAXS) can distinguish between hair from confirmed cancer patients and relatives lacking any cancerous malady. Results brought forth that significantly altered SAXS profile is seen in cases of cancer, tuberculosis, PCOD and NAFL compared to normal ones. We developed programs for automated data collection, analysis and interpretation. Our methodology has been confirmed with about 280 clinically validated cases in India. Recently, we are working on reducing the cost of the analysis instrument, so that our diagnosis can be provided at a lower cost. Research was supported by Grand Challenge Program and CSIR, and now we are open to collaborations for third party validation leading towards regulatory clearances.
CSIR-IMTECH has developed two mass-production ready plate based kits to estimate plasma gelsolin levels in humans. Plasma gelsolin is fast emerging as a health condition biomarker and its repletion in compromised cases is under Phase 1 and 2 trials. While, most other labs including commercial set-ups are focusing on improving bulk production of this protein, IMTECH has developed bonsai versions of this protein by structure-based insights. IMTECH successfully demonstrated the anti-sepsis properties of miniaturized versions in LPS-induced sepsis model of mice (Peddada et al., 2013). This project is part of the objective proposed in 12 FYP BioDiscovery where IMTECH proposed to design mini-proteins or peptides capable of rescuing sepsis condition. The kits which have been developed in IMTECH will help in determining a quantitative measure of the sepsis. Alongside, we are testing the kits viability in predicting cases of preterm birth. Both kits come with a cell phone based application for measuring the gelsolin value and providing a prognosis on the patient status.
Three patents have been filed in this regard and negotiations are underway with commercial partners for mass production and marketing of our kits.
Summary/Background/Description:
A lab-scale process for the preparation of PEGylated-streptokinase has been standardized. The process entails the culturing of E. coli carrying appropriate plasmid DNAs encoding for mutant streptokinase, followed by cell-lysis, refolding to their biologically active states, PEGylation reaction and their isolation by chromatographic means. The PEGylated clot-specific protein display plasminogen activation property as well as few additional superior properties listed below:
Advantages/Value Proposition:
This is a cutting-edge technology that has a truly international potential since it can compete with the most advanced clot-buster drug currently available (TPA, tissue plasminogen activator). The technology will be sufficiently scaled-up in-house so that after transfer to a commercial partner it has a minimum take-off period.
Applications:
A blood clot dissolving life-saver drug which is used for the treatment of myocardial infarction, stroke and other circulatory disorders. These clot-specific variants of streptokinase have been developed by protein engineering with increased half-life, decreased immune reactivity, less systemic plasmin generation and less fibrinogen depletion, and therefore highly promising therapeutic lead molecules with global reach.